Anode and Cathode in Electrolysis
Rothstein Susan Cape Electrolysis. Electrolysis in Piscataway NJ.

Electrolysis Of Copper Sulfate Solution With Impure Copper Anode And Pure Copper Cathode Copper Purification Technology Educat Pure Products Copper Solutions
Oxidation and reduction takes place at the same time.

. In contrasts with a cathode an electrode by which conventional current leaves an electrical device. The electrolysis of acidulated water is considered to be an example of catalysis. A common mnemonic is anode current into the device.
What are the products formed at anode and cathode. During electrolysis the anode loses mass as copper dissolves and the cathode gains mass as copper is deposited. Cathode leaves the electrode current and anode enters the current.
The reason this is difficult is. An anode is an electrode by which the conventional current enters into a polarized electrical device. Answer 1 of 3.
Traditional sulfide metallurgy produces harmful sulfur dioxide and is energy intensive. 43 W Prospect St. The direction of current in a circuit is opposite to the direction of electron flow.
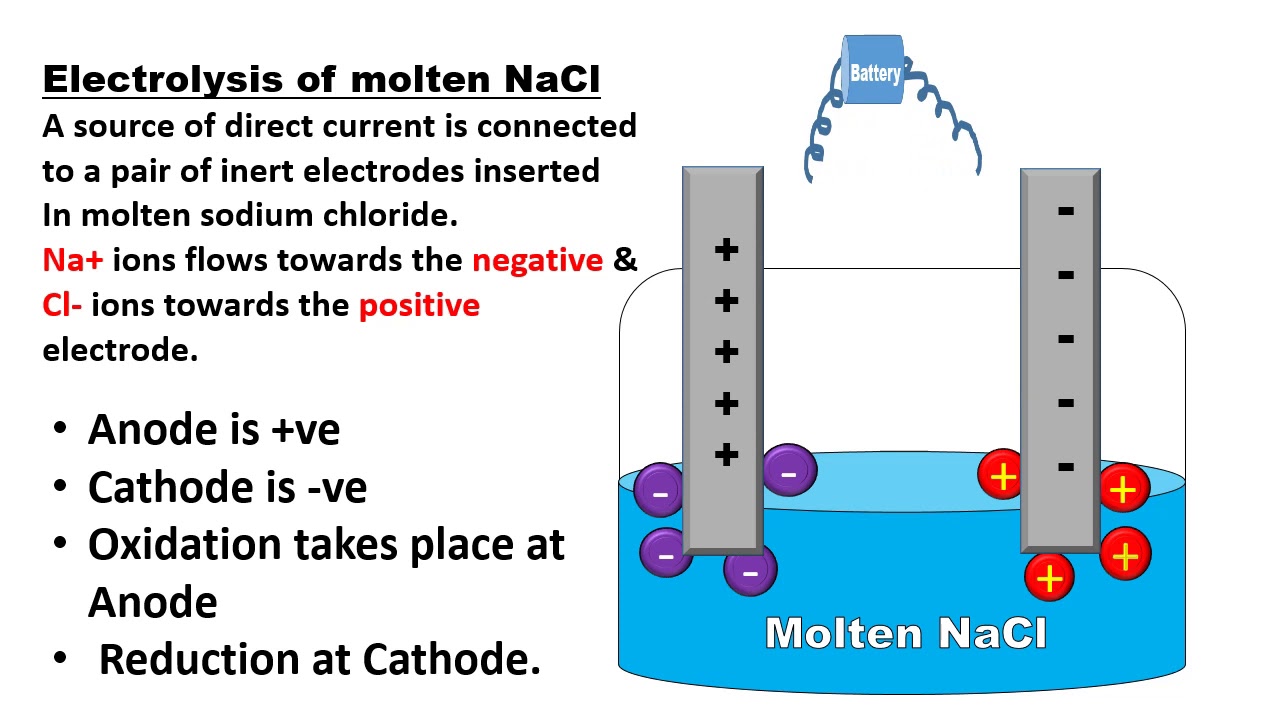
The mercury electrode is common between the two cells. The cathode is the current that leaves the electrodes or the cathode is a result of a reduction reaction taking place in an. The atoms join up in pairs to form Cl 2 molecules so chlorine gas is formed at the positive electrode.
To this end we develop an anode electrolysis approach in molten salt by which sulfide is electrochemically split into sulfur gas at a graphite inert anode while releasing metal ions that diffuse toward and are deposited at the cathode. One or Two 30 or 60-Minute Electrolysis Hair-Removal Sessions at Beauty Factory Spa Up to 65 Off. B During the electrolysis of copper II sulphate solution using platinum as cathode and carbon as anode i State what you observe at the cathode and at the anode ii State the change noticed in the electrolyte.
Up to 10 cash back Discover Electrolysis Hair Removal Deals In and Near West Orange NJ and Save Up to 70 Off. The anode is the negative or reducing electrode from where electrons are released to the external circuit and oxidize during an. The CastnerKellner process is a method of electrolysis on an aqueous alkali chloride solution usually sodium chloride solution.
Anode is electron deficient and hence the negative ions are attracted to the anode where they lose electron and become atoms. The other type of cell shown in the center of the diagram uses an electrolyte of sodium hydroxide solution a mercury anode M and an iron cathode D. Now about anode if cathode is negatively charged so.
You can use pretty much any metal for the cathode the electrode that evolves hydrogen but the anode is more difficult. However limited attention has been paid to the study of cathode. You need something that will resist corrosion while remaining electrically conductive.
Yun et al6 examined the effect of the configuration of the slits formed on the cathode on the escape of the hydrogen bubbles toward the backside of. 70 for One Electrolysis Hair-Removal. The reaction at the anode is oxidation and that at the cathode is reduction.
These factors are similar to those you might use to determine which business to. The reaction at the cathode involves reduction of cations as they gain electrons to become neutral atoms and oxidation takes place at anode as they lose electrons to become neutral. Anode and Cathode.
The so-called anode effects and polarization and to avoid the difficulties related to the carbon anode 3-5. Anode and cathode are defined by the flow of current. One or Two 30 or 60-Minute Electrolysis Hair-Removal Sessions at Beauty Factory Spa Up to 65 Off.
It is the electrodemetal plate that is connected to the positive terminal of the cell. The anode positive electrode is made from impure copper and the cathode negative electrode is made from pure copper. Susan Rosenberg Electrolysis.
What is the substance left at. East Brunswick NJ 08816. SuperPages SM - helps you find the right local businesses to meet your specific needs.
The Cathode is the positive or oxidizing electrode that acquires electrons from the external circuit and is reduced during the electrochemical reaction. The cathode is negatively charged to remember how the cathode is negatively charged consider caThode T as a negative so it is easy to remember carhode as a negatively charged. During the electrolysis of molten salts a metal forms at the cathode and a non-metal forms at the anode.
In the industrial of brine carbon is the anode while mercury is the cathode. A half-equation shows what happens at one of the electrodes during electrolysis. Answer 1 of 3.
Search results are sorted by a combination of factors to give you a set of choices in response to your search criteria. Cathode is the electrodemetal plate that is connected to the negative terminal of the cell. Electrolysis is a redox reaction ie.
The terms were coined in 1834 by William Whewell who derived the words from the Greek word Kathodes which means descent or way down.

Look4chemistry Electrolytic Cell Electrochemistry Cell Positive And Negative

Electrolytic Cell Electrolysis Of Nacl Notas De Quimica Quimica Matematicas

Electrolysis Process On Passing Electric Current The Cations Move Towards The Cathode And Get Deposited Piscinas De Agua Salada Escuela De Natacion Piscinas
What Is Electrolytic Cell Electrochemistry Chemistry Basics Chemistry
No comments for "Anode and Cathode in Electrolysis"
Post a Comment